How CMS Fudged the Math:
If we want reimbursement systems that reflect real-world care and outcomes, we need to speak up. The comment period is now open. Read the full breakdown from John Schroeder and Howard Walthall to learn more about how you can join the call for fair, evidence-based reimbursement.
In the 2026 proposed Physician Fee Schedule and OPPS, CMS proposed to separately pay for skin substitutes in both the physician practice and hospital outpatient department at a rate of approximately $125.38/cm2. Allegedly this figure was based on the actual ASPs of the skin substitutes in the market, weighted by the relative usage of each product. As anyone familiar with the market can readily tell, however, this figure is far lower than the current actual average ASP. To achieve this end CMS engaged in some “funny math,” weighting skin substitute volume based only on hospital usage, and entirely ignoring use in the physician office and mobile practices, two of the primary areas of practice to which this new “rule” would be applied.
CMS tried to justify its decision to ignore actual usage in the physician office based on the claim that office usage was “distorted” by the side effects of CMS’s own reimbursement structures. However, the same is very much true in the hospital outpatient environment. CMS’s bundled reimbursement scheme in the HOPD tends to restrict skin substitute pricing and to limit hospital wound clinics to smaller, less severe wounds which may call for different product choices. Additionally, the hospital GPO and purchasing systems result in slower uptake of new products, meaning that the weighting approach CMS chose tends to strongly favor older products over newer technologies. In practice, GPO awards grant these older products virtually guaranteed high volume which allows the respective manufacturers to sell outdated products at artificially low prices. This approach by CMS thus restricts product innovation by artificially depressing true market dynamics.
We are supportive of the idea of setting consistent reimbursement systems for the office and HOPD, as well as removal of the artificial limit on the treatment of large wounds in the HOPD. That CMS policy has been challenged by clinicians and industry for years now. However when setting a rate, it stands to reason that CMS should take into account product usage in all relevant settings of care.
Speaking of parallel systems, we again support CMS decision to retain a significant facility fee component for the application of skin substitutes in the HOPD, in addition to the payment for the product. This ensures fair reimbursement for the clinical work involved, and reduces the pressure for product pricing concessions to fill the gap and make the application of skin substitutes economically feasible for providers.
However if the PFS and OPPS systems are to be comparable, the clinical fee paid under the physician fee schedule is far too low. Under the current proposal, the HOPD would receive a facility fee of $746.61 plus an additional physician fee of almost $100, while a physician
applying a skin substitute in his or her office would receive about $150 total, over 80% less. Some difference in price structure between the hospital clinic and office certainly makes sense, but this gap is far too large. The fee in the physician’s office should be raised substantially.
So, what is the right number for skin substitute reimbursement for 2026? Based on work from Dr. Tettelbach and others, the value of an average skin substitute to CMS in terms of the avoidance of other care costs such as amputation and readmission (not even mentioning the benefit to the patient) looks to fall in the range of $550-750 per sq cm. Were CMS to do its calculations of current skin substitute average pricing in a straightforward manner, we are confident the result would be at least this high. These are the numbers clinicians and industry should be citing to CMS during the critical comment period we now find ourselves in.
John Schroeder, CEO and Howard Walthall, Senior Vice President of Venture Medical jointly contributed to this article and would like to acknowledge the continuing work of Dr. William Tettelbach, MD.







.svg)



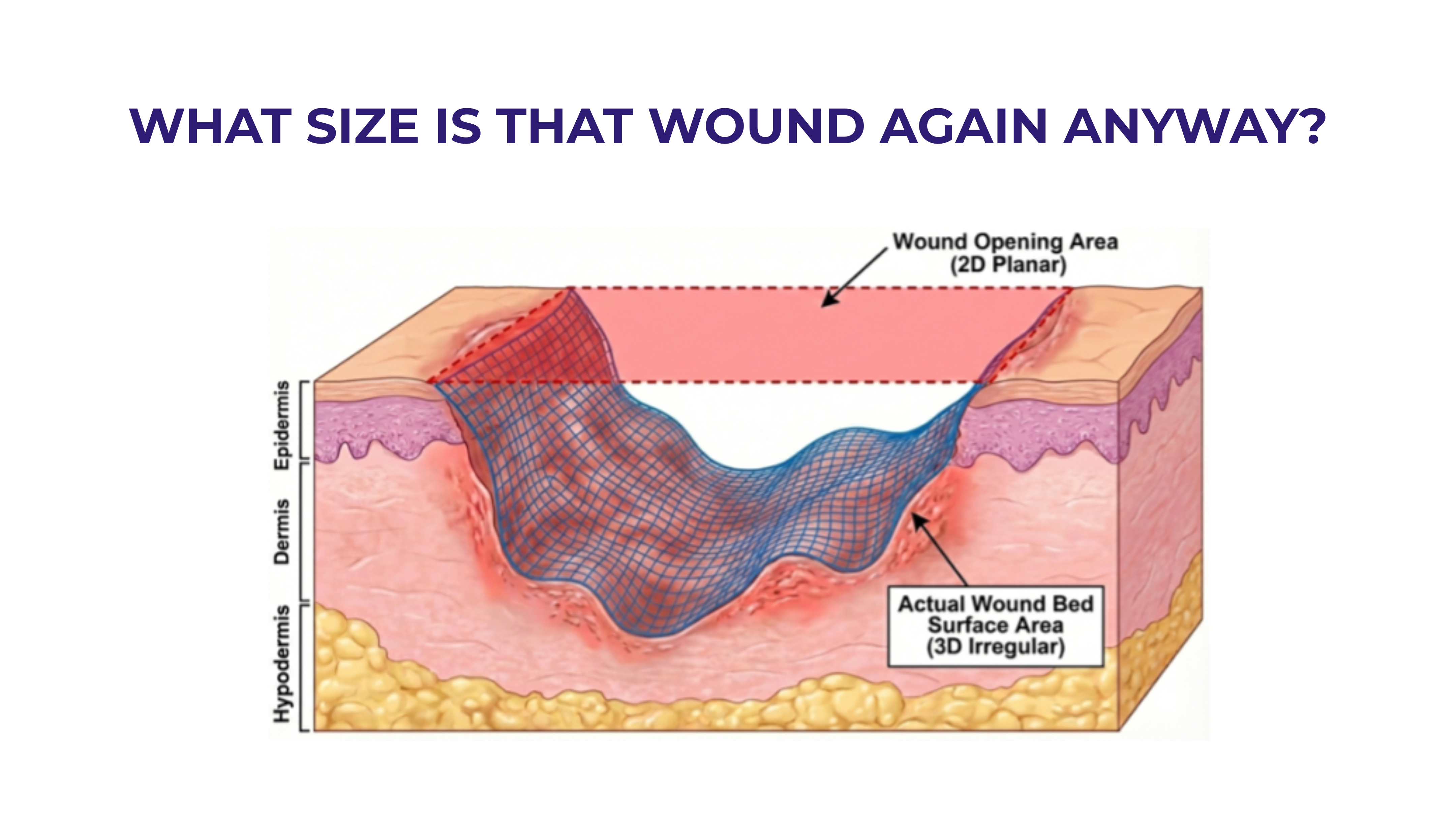

.jpeg)






