The 2026 OPPS and PFS Rules: A Defining Moment for CAMPS Payment Reform

Venture Medical, a leader in wound care solutions, stands at the forefront of advocating for patient access and innovation in response to the 2026 Hospital Outpatient Prospective Payment System (OPPS) proposed rule.
Executive Summary
The long-anticipated publication of the 2026 Hospital Outpatient Prospective Payment System (OPPS) and Physician Fee Schedule (PFS) proposed rules marks a watershed moment for CAMPS reimbursement. The expected introduction of a new payment system for CAMPS products represents the most significant policy shift in wound care reimbursement in over a decade. The industry's response during the critical 60-day comment period will determine whether this transformation advances patient access or creates new barriers to innovative care. Success demands unprecedented coordination, leveraging robust value-based evidence and strategic National Coverage Determination (NCD) requests. The issue at hand is not simply a dramatic price reduction in CAMPS, although the rumored $130 a cm is severe and well below CMS’s break even with the expected cost of increased negative clinical sequalae costs. These were identified separately by Tettelbach et al and Marissa Carter at about $450/sq cm. (In fact, we expect these numbers to rise closer to $600/cm when updated analyses of 2024 CMS claims data are completed very soon). The system breaking even versus the monetary cost of under treatment is far from the only concern, however. There is an enormous human cost to relying on amputations, ER visits, systemic antibiotics, and other last ditch efforts to mitigate the life-threatening consequences of undertreated chronic wounds. At the business end of the past several years of CMS coverage and price wrangling is the 10-15 million potentially lost limbs and lives. Adding insult to injury, they created the broken system and, so far, have refused numerous suggestions from OIG and even The White House to fix it. Patients are the ones who will suffer more than any changes to CMS’s budget lines can ever show. Put into context, there are 10 pharmaceuticals that cost the healthcare system more than $10 Billion annually. The total spent on CAMPS this year, although outrageously high and agreeably in need of reduction, is less than just one of those drugs.
The Shifting Payment Paradigm
Current State Analysis
The existing ASP+6% system applied to products with low barriers to entry from FDA’s section 361 approval process has provided a foundation for CAMPS reimbursement. Low barrier as opposed to multiple RCTs before marketing has become alarmingly inadequate and a moral hazard for industry and providers alike. The expected proposed rules introducing a new payment system for CAMPS products within the Physician Fee Schedule will signals CMS's recognition that these innovative therapies require a more unique payment approach that better reflects their clinical value and pricing administration complexity in this FDA versus CMS regulatory mismatch. Yes, “ASP+6” has become unsustainable. No efforts to date by colluding Medicare Administrative Contractors (MACs) to restrict access through highly biased coordinated LCDs and the “guilty until proven innocent” draconian audit scrutiny have worked to impede the alleged fraud, greed, or explicit price escalation in this market.
Market Implications
The 2026 rules’ new CAMPS payment system will fundamentally alter the economic dynamics driving how CAMPS are delivered, and in what place of service. This could create large disparities between patient groups in the real world. Many of the CAMPs are utilized on patients in outpatient environments, often in patient homes and nursing facilities. Variables that will drive inequities include rural versus urban, high versus low socioeconomic status, education and awareness levels, and age-related stratifications. Chronic wound patients are predominantly elderly and members of minority groups; the majority of whom are driven by cost savings to Medicare Advantage plans that grossly ration CAMPs use even as they see widespread clinical utility in direct Medicare part B beneficiaries.
If the new flat reimbursement rate that is set is too low, healthcare systems and physicians will need to reassess their service offerings. The reimbursement may no longer allow for providers to go to where these patients reside as they have been. Manufacturers must recalibrate their market strategies, likely forcing many of them out of the chronic wound market. Additionally, many clinicians who continue to see wound patients may find it no longer economically possible to provide skin substitutes and other advanced therapies to these patients, resulting in either increased treatment deferrals or denials of needed care.
What will result are product and service location oligopolies characterized by long wait times and suboptimal frequency of care provision with the cheapest products as many more patients and their caregivers will now have to navigate strong economic and logistical barriers to HOPD clinic-based care. At the same time, hospital outpatient clinics could be rapidly overwhelmed, with wait times for appointments rising beyond the typical 4-8 weeks now present in many markets. Imagine if a patient is poor, living in a nursing home that does not have a high enough mix of private payors to offset the low reimbursement incurred by Medicaid and Medicare payments: They will find it very difficult to convince administration to provide regular medical transportation to the clinic which can cost several hundred dollars a day. The transition period with inefficient providers leaving the market will likely create massive provider shortages as well. Early adapting provider groups who are already highly efficient and use state of the art business analytics and AI for everything from scheduling, charting, billing and revenue cycle are few and far between. Those who align with this new framework embracing these tools will gain significant competitive advantages over time, but in the interim, patients will suffer.
Thus, the rate per square centimeter cannot go below the supposed CMS break-even point, even temporarily, and must arguably remain above $700 a cm so that enough providers and practices can continue to care for the approximately 12 million patients with chronic wounds. To complicate this, although patients can re-elect Medicare B, this is a relatively unknown process, so the 60% of those under a Medicare Advantage plan which will deny payment for the vast majority of CAMPs claims will continue to go without. This disparity is again easily remedied by an NCD. Further reductions in CAMPS pricing cannot happen in an environment where human suffering does not exponentially rise until an NCD providing access for patients with all
types of chronic wounds to receive CAMPs therapies is enacted. In fact, the MACs know this and avoided the NCD process with restrictive and coordinated LCDs because there are many financial ties between them and the very insurance companies who sell the Medicare Advantage plans to vulnerable patients. This incestuous relationship is in fact the real issue and further delay in extinguishing their efforts to restrict access to care therefor cannot be unwound from CAMPs pricing reform. Thus, the price reductions must be adjusted concurrently with an NCD.
Strategic Response Framework
1. Data-Driven Advocacy
The 60-day comment period presents a critical window for industry stakeholders to influence the final rule. Success requires:
Clinical Evidence Compilation: Aggregating robust outcomes data demonstrating CAMPS efficacy, safety profiles, and cost-effectiveness compared to conventional treatments. This evidence must be presented in formats that directly address CMS's coverage criteria.
Real-World Evidence Integration: Collecting and presenting post-market surveillance data that demonstrates consistent clinical benefits across diverse patient populations and practice settings.
Economic Impact Analysis: Quantifying the broader healthcare system impacts, including reduced hospital readmissions, decreased need for subsequent interventions, and improved patient quality of life metrics. Critical value analysis data published by Dr. Bill Tettelbach in the Journal of Wound Care demonstrates the compelling economic case for CAMPS products in diabetic foot ulcer management. When patients received debridement at intervals of 7 days or less with concurrently applied skin substitutes, observed amputation rates dropped by 65% with the lowest levels identified among Medicare LEDU episodes (2.1%). This dramatic reduction in amputation rates translates to substantial Medicare cost savings, as the cost of amputations can be 10 to 40 times greater than the initiatives to prevent them.
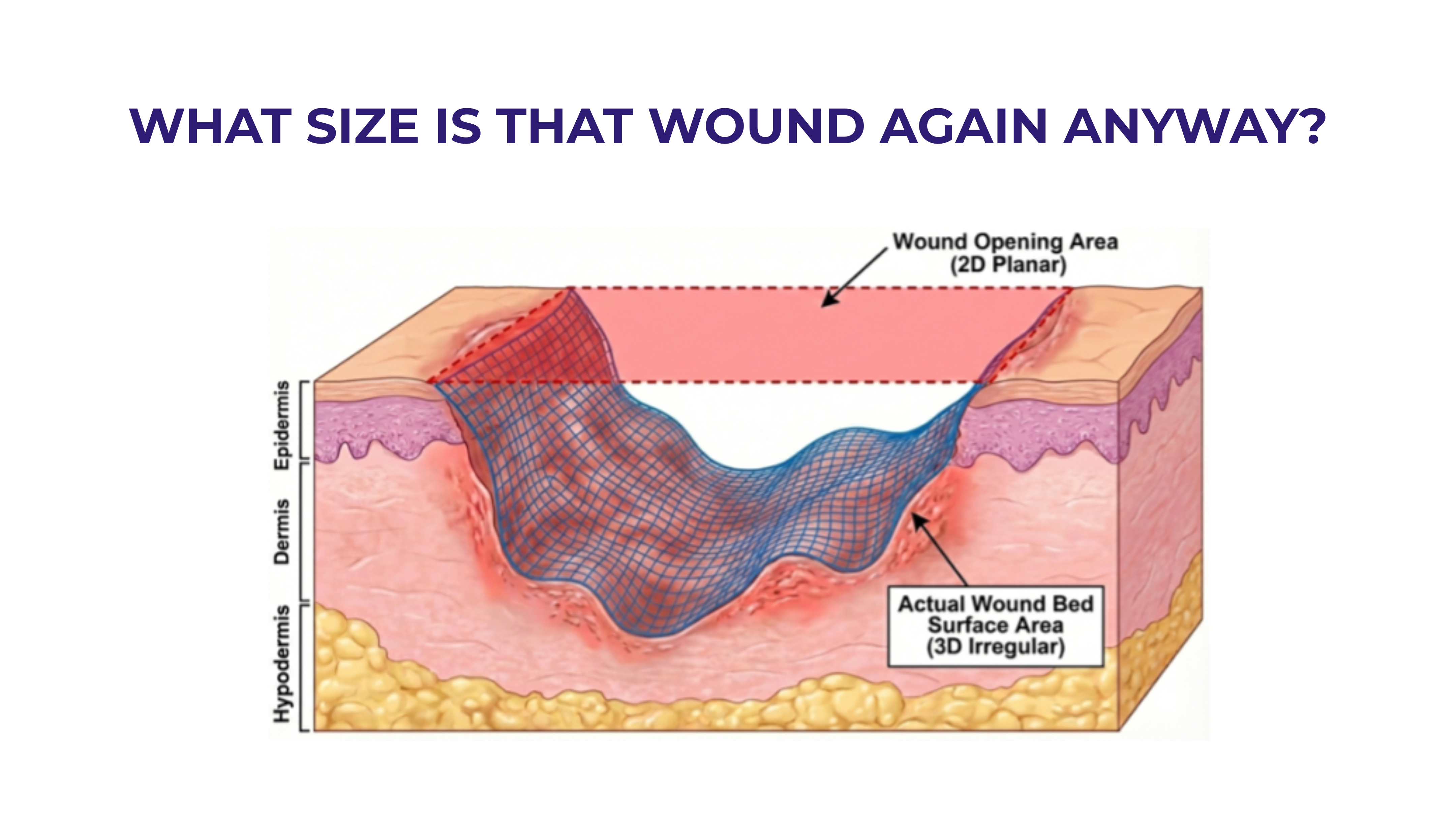
Cost-Per-Square-Centimeter Value Framework: The industry must address the current pricing disparities in CAMPS products, where pricing per square centimeter varies from $30/cm2 to nearly $5,000/cm2 for the latest product added to the ASP file. However, this cost analysis must be viewed through the lens of amputation prevention value, where the Medicare cost of a major lower extremity amputation far exceeds the investment in advanced wound care therapies. Advanced treatment for the management of LEDUs was associated with significant reductions in major and minor amputation, ED use, and hospital readmission compared with LEDUs managed with standard care.
Revealing Hidden Costs of Access Limitations: Analysis published in the Journal of Wound Care examining "The hidden costs of limiting access: clinical and economic risks of Medicare's future effective cellular, acellular and matrix-like products (CAMPs) Local Coverage Determination" reveals the substantial downstream economic burden when Medicare beneficiaries are denied access to advanced wound care therapies. The study demonstrates that restrictive Local Coverage Determinations (LCDs) create a cascade of clinical and economic
consequences that far exceed the upfront cost of CAMPS products. When patients with chronic wounds cannot access appropriate cellular, acellular, and matrix-like products, they experience prolonged wound healing times, increased infection rates, higher hospitalization frequency, and ultimately higher amputation rates.
Performing Medicare Burden Analysis: The economic modeling shows that each delayed or denied CAMPS treatment results in an average increase of $47,000 in total Medicare costs per patient episode, driven primarily by emergency department visits, hospital readmissions, and major amputations. This represents a false economy where short-term coverage restrictions generate exponentially higher long-term costs. The analysis further demonstrates that patients subject to restrictive LCDs experience wound healing rates 40% slower than those with appropriate access to advanced therapies, directly correlating with the increased utilization of high-cost acute care services.
2. Coordinated Industry Response
The fragmented nature of previous industry responses to payment policy changes has limited effectiveness. This cycle demands unprecedented coordination:
Multi-Stakeholder Coalition Building: Uniting manufacturers, medical societies, patient advocacy groups, and healthcare providers under a unified strategic framework.
- Synchronized Comment Submission: Developing complementary rather than duplicative comments that collectively present a comprehensive case for favorable payment policies.
- Thought Leader Engagement: Leveraging respected clinical experts to provide authoritative clinical perspectives that validate industry positions.
3. National Coverage Determination Strategy
The NCD pathway offers the most direct route to ensuring appropriate coverage for CAMPS products:
Broad Indication Approaches: Developing separate NCD requests for distinct clinical applications, each supported by indication-specific evidence packages would be a traditional approach, but the differences in all types of chronic wound dissipate with pathological examination of the tissue is performed across all etiologies and it is discovered that repetitive ischemic injury is common to all of them. The somewhat artificial division by etiology approach dates to very early clinical trials which sought to control included patients' study populations for the study sponsor’s convenience and assurance of positive statistical outcomes. Those study populations have never and will never represent the patients treated in the real world. For example, recent registry data yet to be published will show a profound difference between stage 4 pressure ulcers treated with CAMPs versus match cohort treated with standard of care, thus defying the conventional wisdom and existing LCD guidelines that CAMPS are only supposed to be used on simple DFUs and VLUs.
Comparative Effectiveness Research: Commissioning studies that directly compare CAMPS interventions to current standard-of-care treatments, focusing on endpoints most relevant to CMS decision-making.
Patient Access Arguments: Demonstrating how current coverage limitations create barriers to optimal patient care and health equity.
4. Congressional Legislative Strategy
Given the urgency of wound care access issues and the compelling economic data demonstrating CAMPS value, the industry should pursue parallel legislative action to accelerate NCD development:
Key Congressional Engagement: Engaging key House and Senate members, including key members of the House Ways and Means Committee and the Senate Finance Committee to champion comprehensive wound care coverage reform in a broad-based NCD. Financial savings information needs to be demonstrated at several pricing levels alongside validated statistical survey results showing how many providers will be forced to restrict the availability of skin substitutes for their patients or exit the wound care practice entirely based on the severity level of the price cuts. The cost savings information from CMS claims data is already being finalized, and the surveys have already been commissioned by expert polling practitioners. While these committees have jurisdiction over Medicare policy and can influence CMS priorities through oversight and legislative action, only a dedicated bill will compel an NCD and appropriate pricing action in time to prevent massive suffering and a concurrent industry and physician exodus from the wound care field.
Targeted Legislative Framework: Drafting legislation that would compel CMS to initiate the NCD process immediately for all types of wounds, establishing clear timelines for coverage determinations. This approach recognizes that the current ad hoc coverage system leaves critical gaps in wound care access, particularly for Medicare beneficiaries with diabetic foot ulcers and other chronic wounds.
Bipartisan Coalition Building: Leveraging the compelling amputation prevention data to build bipartisan support, emphasizing how immediate NCD initiation would protect Medicare beneficiaries from preventable amputations while generating long-term cost savings for the program. The 65% reduction in amputation rates demonstrated in Dr. Tettelbach's research provides a powerful foundation for congressional advocacy.
State Delegation Outreach: Engaging senators and representatives from states with high diabetes and wound care burden to champion the legislation, using state-specific data on amputation rates and Medicare costs to demonstrate local impact. In addition, the Doctor’s Caucus, Health Services committee members, and others must be targeted as cosponsors. Access does not just come from political donations, but from direct coordinated efforts from those who may be most aggrieved. Physicians and patients must also be given easy methods to communicate, such as the fairwoundcare.org website.
Implementation Roadmap
Phase 1: Immediate Response (Days 1-20)
- Compile existing clinical evidence and identify data gaps
- Self-assemble aligned industry, physician, and practice leaders
- Initiate stakeholder outreach and coalition building efforts
Phase 2: Evidence Development (Days 21-40)
- Complete comprehensive literature reviews and meta-analyses
- Engage health economists for cost-effectiveness modeling
- Conduct physician surveys on clinical utilization patterns
- Develop patient case studies demonstrating clinical impact
- Initiate congressional outreach to key committee leadership
- Begin drafting legislative language for NCD acceleration
Phase 3: Submission and Advocacy (Days 41-60)
- Submit coordinated comment letters addressing key policy concerns
- File formal NCD requests with supporting evidence packages
- Engage in direct dialogue with CMS officials and contractors
- Coordinate media outreach to raise awareness of patient access issues
- Present legislative proposals to congressional leadership
- Mobilize patient advocacy groups for congressional support
Conclusion
The 2026 OPPS and PFS proposed rules’ introducing a new CAMPS payment system represents a defining moment for regenerative medicine. Their publication, expected any day, will starts the clock on a 60-day window that will largely determine whether these innovative therapies receive appropriate recognition and reimbursement under Medicare. Success requires unprecedented coordination, rigorous evidence of development, and strategic deployment of resources.
The opportunity exists to establish a payment framework that supports continued innovation while ensuring appropriate patient access in an economical manner for Medicare. However, this outcome is not guaranteed and will only be achieved through sustained, coordinated action that prioritizes patient needs and clinical evidence over narrow commercial interests.
The stakes are high, but so is the potential for transformative change. We must seize this moment to shape a payment system that supports the promise of CAMPS for millions of Medicare beneficiaries.
Call to Action
Healthcare industry stakeholders must act decisively:
- Engage immediately in coordinated response efforts
- Build strategic partnerships across the healthcare ecosystem
- Mobilize congressional allies to champion NCD acceleration legislation
- Maintain long-term perspective on policy evolution
- Prioritize patient access in all advocacy efforts
The future of CAMPS coverage depends on actions taken in the coming weeks. The industry cannot afford to let this opportunity pass without maximum coordinated effort across both regulatory and legislative channels.



.svg)




.jpeg)





